International cooperation is alive. COVID-19 vaccine development will benefit from years of global collaboration on seasonal flu vaccine pandemic preparedness.
Ayelet Haran
Trade Vistas Contributor
A COVID-19 vaccine will follow flu footsteps
The global coronavirus outbreak has upended the lives of billions around the world. As anxiety levels remain high and the economy is in free-fall, it remains unclear how we will return to “normal life” in the short term.
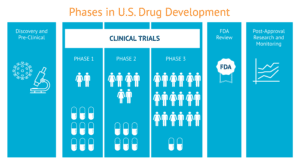
In the long term, vaccine development is our best bet for a future free of COVID-19. Several companies around the world have already launched vaccine discovery with unprecedented speed. Some were able to begin small clinical trials as soon as mid-March, though expectations should be calibrated as the clinical trials process to test safety and immune response is a lengthy one.
The race to a coronavirus vaccine is emblematic of the balance between competition and collaboration, both routine and natural for the global health community. After all, experts around the world collaborate each year to develop, produce and deliver the influenza vaccine, also known as the flu vaccine, to billions of people. They have been working together this way for decades.
The first flu vaccine
According to an article published in the Journal of Preventive Medicine and Hygiene, the earliest confirmed flu pandemic on record first appeared in 1580 in Asia and Russia. It spread from there to Europe and northwest Africa.
Yet it wasn’t until the 1940s when University of Michigan researchers developed the first inactivated flu vaccine using fertilized chicken eggs, still the primary method for making commercial vaccines today.
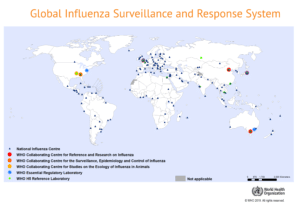
Tracking global strains for drug development
The flu virus changes annually, making it unpredictable. Developing an annual vaccine is the product of a globally-educated guess.
Members of the World Health Organization (WHO) established a surveillance system in 1952 to monitor the emergence of different strains of influenza that have the potential to become a pandemic. The Global Influenza Surveillance and Response System (GISRS) comprises an international network of national laboratories in more than 100 countries. These labs conduct surveillance and share information (including representative viruses) with five WHO centers located in the United States, UK, Australia, Japan and China.
Twice a year, in preparation for the flu season in the northern and southern hemispheres, the GISRS centers convene with representatives from public health bodies, leading research institutions and private sector experts to evaluate and recommend strains to include in the seasonal vaccine. Ultimately, each country decides for itself which viruses will be included in the flu vaccine they license that year.
A network for pandemic preparedness
The WHO also maintains a Pandemic Influenza Preparedness Framework, known as PIP, which includes member governments, vaccine manufacturers, and other stakeholders.
The PIP Framework governs the sharing of virus strains and PIP-related biological materials across organizations and borders, which is critical for determining which virus strains manufacturers should target in the seasonal vaccine. The Framework also coordinates access by the world’s most vulnerable populations to vaccines and treatments and manages agreements on intellectual property and licensing of the vaccine.
PIP’s industry partners receive access to the virus strains they need to make the vaccine. In exchange, they agree to provide benefits to the WHO and to developing countries in the form of vaccine donations, royalty-free licensing to manufacturers in low-income markets to make the vaccine, or a guarantee of a specified quantity of vaccine supply at lower prices, among other arrangements.
 Vaccine production and distribution
Vaccine production and distribution
Once the most dangerous virus strains for the upcoming seasonal flu have been selected, manufacturers turn to producing the vaccine. According to Sanofi Pasteur, the top global producer of the seasonal influenza vaccine, it takes between 6 and 36 months to manufacture, package and deliver high-quality vaccines to those who need them.
The viruses are first grown in a lab setting, after which the antigens are extracted from the viruses and purified to eliminate any raw material traces. Next, the virus goes through an in-activation process that retains the properties that will elicit an immune response in the body. Next, the active substances are combined into a single chemical component. These chemical components can be combined with others to form a single shot, like the MMR vaccine that includes compounds inoculating against measles, mumps and rubella. The vaccines are then filled into a vial or syringe, packaged and shipped all around the world.
Vaccine makers have to produce several different vaccines to meet the strain selection of each country. For example: CSL, another leading influenza vaccine maker, produced seven different influenza vaccines for the last flu season.
Moon shot against any flu virus
The laborious process of monitoring, surveilling, selecting, and then inoculating against specific flu virus strains, still leaves the possibility that an unexpected strain will emerge and result in a pandemic. Some seasonal flu vaccines are not an effective match against the strain that emerges. These lingering uncertainties have motivated the public health community to work towards making a universal flu vaccine – one that could provide long-lasting protection for multiple strains of influenza in one shot.
The potential for such a shot has captured the imagination of world leaders and influencers, including Bill Gates who committed millions in grants to this research. President Trump signed an Executive Order on September 19, 2019 that directs the U.S. Department of Health and Human Services to promote new vaccine manufacturing technologies and advance the development of vaccines that provide longer-lasting coverage against a broad range of flu viruses.
Researchers have seen some success. One example: early trials focused on proteins in the flu virus that remain stable and activate an immune response to stop and destroy the infection. While we are still years away from having access to such a vaccine, there is some momentum building behind this approach.
The here and now vaccine
We don’t know how soon we could have a vaccine against COVID-19. But we do know that COVID-19 vaccine development is benefiting from years of global collaboration on seasonal flu vaccine pandemic preparedness. This includes sharing biological resources, disseminating data and research, and coordinating manufacturing rights and distribution.
Is it a perfect system? No – but doctors, scientists, labs, drug companies and national health institutions have experience in what it takes to bring the world’s knowledge to bear in a well-coordinated framework, which could speed up the discovery of a COVID-19 vaccine.
*This article was originally published on TradeVistas and is subject to their Reprint Policy and Copyright Notice. It is provided here with TradeVistas’ permission and knowledge.